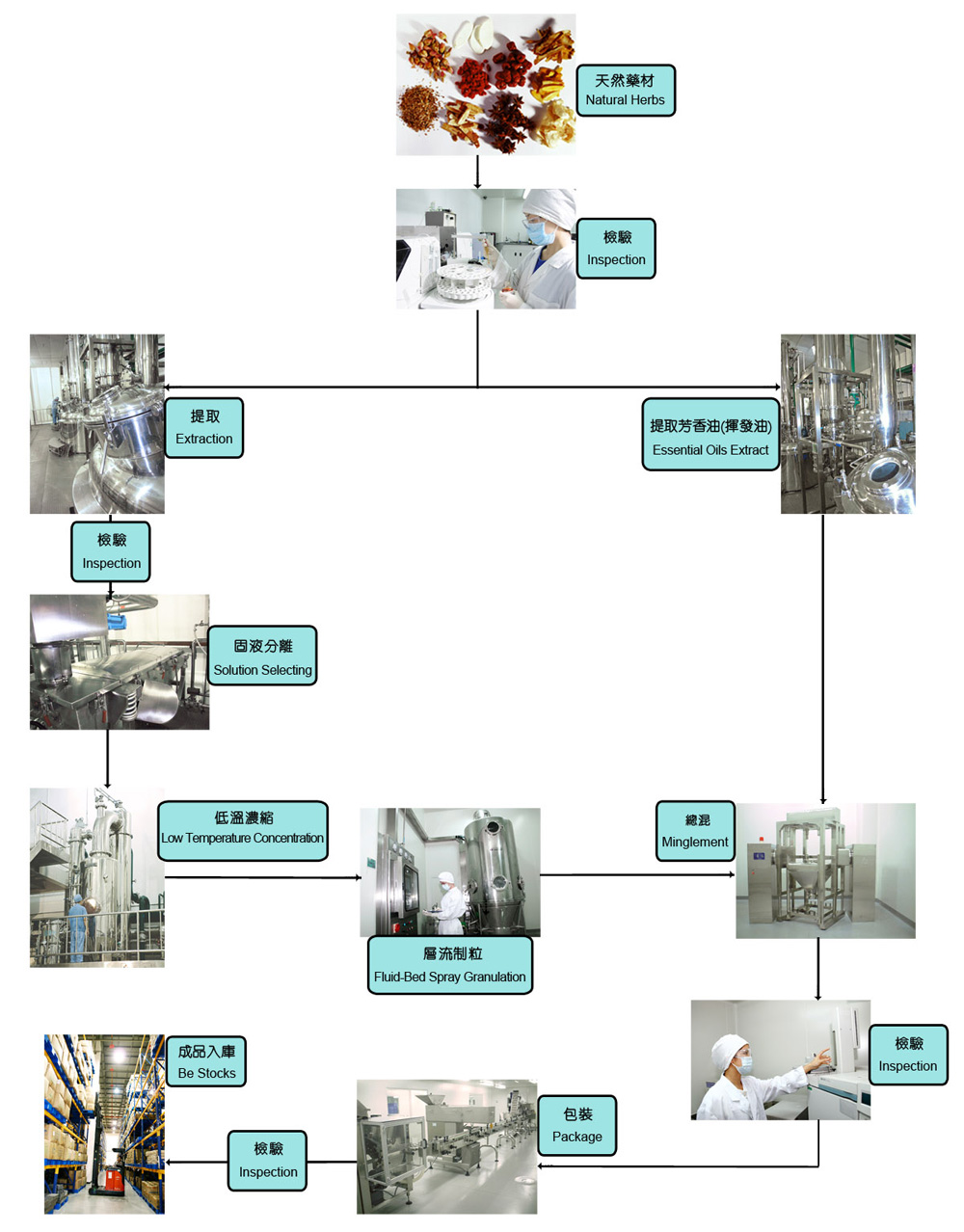
We have strict standard on all products, each batch of products undergoes strict quality inspection. All testing laboratories meet the highest national standards. We use imported instruments for inspection, including: high performance liquid chromatography, atomic absorption spectrometer, infrared spectrometer, thin layer chromatography scanner, gas chromatograph, etc.